Photo courtesy of Gerdus Bronn | ||
Finding the Hidden Sun with this Remarkable Stone |
||
| Update 12/26/15: For an in-depth look at the optical properties of iolite and pleochroism which make it suitable as the Viking Sunstone, see: Skalwold, E.A. and W.A. Bassett. (2016) Blue minerals: exploring cause & effect. Rocks & Minerals, Vol.91, No.1, pages 61-75 (abstract). Iolite is the beautiful mineral cordierite which exhibits eye visible pleochroism as it is rotated in the light (shown in the above photos, in these fashioned iolite cubes and explained further below). It is the gemstone most often associated by the public with the Viking Compass story. Therefore it is fun to explore the properties which might lend themselves to a workable model. On the question as to just how one would use iolite to find the hidden sun, the answer is similar to how Leif Karlsen employed calcite. He observed two images through the calcite, one of which lost intensity or tone when moved off of a direct line with the position of the sun. Though one does not see two images (doubling), the strong pleochroism of iolite can be used in the same manner. Both doubling and pleochroism can be properties of anisotropic minerals. ("a crystal must be anisotropic in order to show pleochroism and a crystal must be anisotropic to show birefringence. But a crystal needn't be birefringent in order to show pleochroism. It could have a birefringence of zero and still show strong pleochroism."W.A. Bassett) | ||
The Axial and Face Colors of Iolite |
||
| In the following, keep in mind that the path of light is different than the vibration direction: it may travel parallel to the c axis, but its vibration direction is perpendicular to that path. "Iolite (cordierite) is an example of a mineral with extreme pleochroism (color which varies with the polarization orientation of the incident light - see more). Because iolite is orthorhombic, there will be three independant absorbtion spectra obtained with light linerally polarized along the a,b, c-axes. Most important for determining the visible color is the intervalence charge transfer process (IVCT)" (G. Rossman). Light polarized parallel to the c axis may be yellow (or almost colorless) due to isolated Fe+3 ions; there is no IVCT in this direction. "Color and pleochroism probably arise from (this) IVCT between the octahedral Fe+2 and channel Fe+3." The stongest blue color in iolite occurs when the direction of polarization is parallel to the b crystollographic axis ("beta" or "macro").(B. Amos). | ||
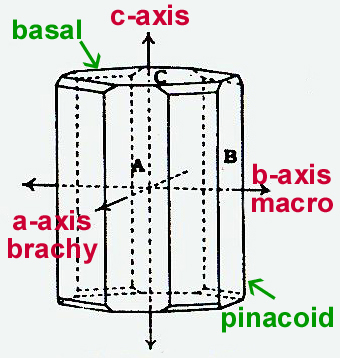 Adapted from H.A. Miers, 1902. As with calcite, when light enters the iolite crystal, it is broken up into two plane polarized rays (mutally perpendicular to each other). The two rays absorb different wavelengths of light differentially, making each ray appear a different color. When they exit the crystal you see the colors of the two rays combined into one. With a deceptively simple instrument called a dichroscope, you can observe these two colors separately, side by side. This is achieved by looking through the dichroscope while holding the the stone in various orientations against the light. By the way, inside the dichroscope there is a nice peice of optical quality calcite, just like the one Karlsen used. The calcite displays the two colors by seperating the rays just as it shows two images of a dot when looking through it. Karlsen made use of the dot; here we use the pleochroic colors. Note: actual colors seen in a specific crystal may be different than those given below due to varying chemical make-up of different samples. Classic references often cite pale yellow, deep blue-violet, pale blue. The axial colors as seen through the dichroscope:
| ||
 Iolite Pebbles Over Polarizing Filter. Photo courtesy of Dr. G. Rossman |
In the photo to the left, crystals of iolite are seen placed over a linear polarizing filter so that the vibration direction of the light is parallel to the different crystallographic directions. Darkest blue = E\\ β = b = 9.7 ångstroms Medium blue= E\\ γ = a = 17 ångstroms Pale stone = E\\ α = c = 9.3 ångstroms. (Dr.Rossman, personal communication. Note: in his Sp. 1988 G&G paper, p. 7, the colors for vibrations \\ to a and b are reversed from scenario above). | |
If the light entering the stone is already polarized, or partially polarized such as that coming from the low sun (caused by scattering), the color seen in the iolite will be intensified when correctly aligned in the direction of maximum polarization, that is, the zenith.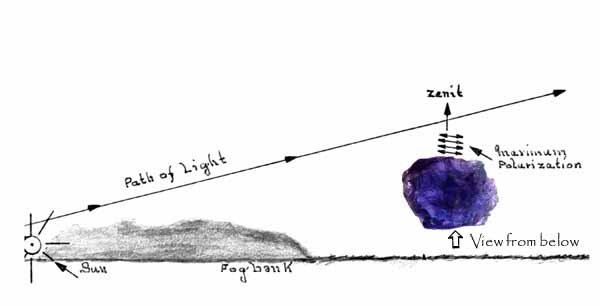 Adapted from L. Karlsen with apologies to the original artist. See original here. Iolite is the mineral most popularly associated with the theory of the Viking Compass or Sunstone and it was the one which Thorkild Ramskou strongly favored. Leif Karlsen played with the idea of using pleochroism; he too wanted iolite to be the Viking Sunstone, but he could not obtain satisfactory navigational accuracy using it. The problem wasn't determining the maximum saturation of the pleochroic color, the difficulty was knowing what to do with that knowledge. Once one determined the maximum "color change," the question remains: in which direction was the hidden sun? There needs to be a readily available direction indicator with the stone. With calcite, the straight sides of the crystal line up exactly with the sun, thereby providing a bearing. The iolite pebble would have to be set in a frame of wood or metal with bearings indicated, or the stone somehow permanently marked in some fashion, then the device would have to be calibrated to the bearings of the sun to give a useful reading. Once it was calibrated, it would probably work very well (June Karlsen, personal communication). Calibration would entail determining the long axis of the crystal fragment; not so easy to do without the right tools, such as a dichroscope. Now, one could pick up a piece of calcite, wrap it in something dark and make a rudimentary dichroscope with which to view the axial colors of the iolite, but were my Viking ancestors that inquisitive? perhaps.... | ||
| ||
| For more on Viking Navigation and the work of Leif Kalrsen using calcite, please go to the Karlsen section of this website. | ||